Abstract
Introduction: Inotuzumab ozogamicin (InO) is a CD22-directed antibody-drug conjugate indicated for the treatment of relapsed/refractory (R/R) B-cell precursor acute lymphoblastic leukemia (ALL). InO has been associated with hepatotoxicity and hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS), particularly following hematopoietic stem cell transplantation (HSCT). This study examined post HSCT outcomes in patients who received InO prior to HSCT.
Methods: This observational, post-authorization safety study included patients in the United States with B-cell precursor ALL who were treated with InO and received subsequent HSCT at a participating center, and whose data were reported to the Center for International Blood and Marrow Transplant Research. We report outcomes in adults (aged ≥18 years) with ALL, and the subset of adults with R/R ALL, who received InO and proceeded to first allogeneic HSCT. Post HSCT outcomes included disease status, overall survival, non-relapse (transplant-related) mortality (NRM), non-transplant-related mortality, and adverse events (AEs) of interest, including VOD/SOS. Multivariate analyses examined prognostic factors of NRM at 1 year and VOD/SOS incidence at 100 days post HSCT. This analysis is based on 4-year interim data, accrued from August 18, 2017, to August 17, 2021 (data lock: September 30, 2021). The study is ongoing and will continue for a total observational period of 5 years.
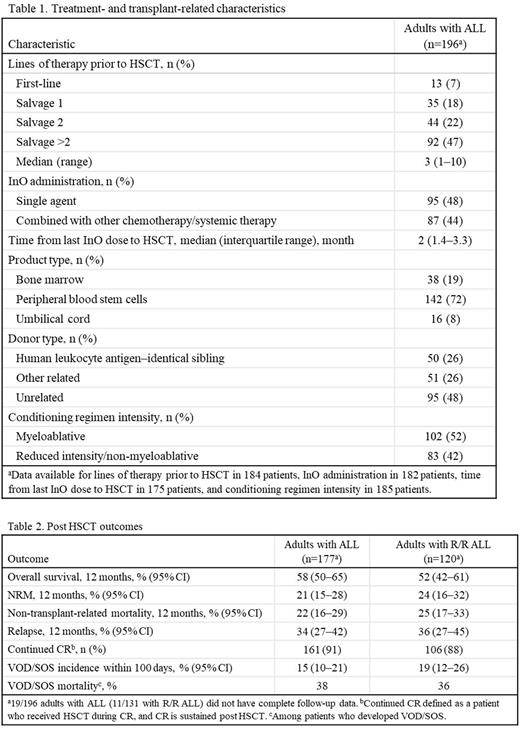
Results: There were 196 adults (median age 39 years, 54% male) with ALL who received InO and proceeded to first allogeneic HSCT: 32% in first complete remission (CR1), 46% in CR2, 12% in CR≥3, 5% in first relapse, 2% in third or subsequent relapse, and 3% with primary induction failure (data unavailable in 1%). Prior to HSCT, 32%, 45%, and 17% received 1, 2, and ≥3 cycles of InO, respectively (data unavailable in 6%). Additional treatment- and transplant-related characteristics are presented in Table 1.
Post HSCT outcomes are shown in 177 adults with ALL, and 120 adults with R/R ALL, with complete follow-up data at the data lock (Table 2). Median (range) follow-up was 8.4 (0.4-39.2) and 7.1 (0.4-38.8) months in the respective groups. The most common causes of NRM at 12 months were VOD/SOS (10/35; 29%) and graft-versus-host disease (GVHD; 8/35; 23%) in patients with ALL. AEs occurring in ≥30% of patients within 100 days post HSCT were bacterial infection (46%), viral infection (41%), and acute GVHD (grades II-IV; 38%). VOD/SOS incidence within 100 days post HSCT was 15% and 19% in patients with ALL and R/R ALL, respectively; corresponding mortality rates were 38% and 36% in patients who developed VOD/SOS within 100 days (6% and 7% as a percentage of all patients). Median (range) time from HSCT to VOD/SOS was 0.4 (0.2-2.6) months in both groups. Of 26 adults with ALL who developed VOD/SOS, 10 cases were mild and 16 severe; 5/26 patients received prophylactic defibrotide; and 12/26 patients received defibrotide treatment either alone or in combination with other treatment. In those who died, median (range) time to death was 4.5 (0.4-39.2) months.
A total of 143 adults with ALL who were alive at the date of last contact and reported ≥1 year of follow-up or who died at any time after HSCT were included in multivariate analyses. Total serum bilirubin at or above the upper limit of normal before HSCT (hazard ratio [HR], 5.72; P=0.0001) and 4 cycles of InO (HR, 6.81; P=0.0009) were negative prognostic factors for NRM at 1 year. A conditioning regimen containing dual alkylators was the only negative prognostic factor for VOD/SOS incidence at 100 days (odds ratio, 8.3; P=0.0033).
Conclusions: In this real-world population of adults with ALL who received InO before HSCT, including heavily pretreated patients, the incidence of VOD/SOS after HSCT was similar to that observed in the phase 3 INO-VATE study (23%; Kantarjian et al, Cancer 2019;125:2474-87) and a pooled analysis of 2 clinical trials of InO-treated patients with R/R ALL (19%; Marks et al, Biol Blood Marrow Transplant 2019;25:1720-29). Although the VOD/SOS mortality rate was higher (36% vs 26%), NRM at 12 months was lower (24% vs 38%) here vs the pooled clinical trial population.
Disclosures
de Lima:Amgen: Consultancy; Incyte: Consultancy; Celgene: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Kebriaei:Amgen: Research Funding; Ziopharm: Research Funding; Pfizer: Consultancy; Kite: Consultancy; Jazz: Consultancy. Lanza:Amgen: Consultancy; Abbvie: Consultancy; Pfizer: Research Funding; Alexion: Consultancy. Popradi:Merck: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Servier: Consultancy, Honoraria, Speakers Bureau; Jazz: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria, Speakers Bureau; Amgen: Honoraria; Syndax Pharmaceutical: Research Funding; Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Taiho: Consultancy, Honoraria; Kyoma Kirin: Consultancy, Honoraria, Speakers Bureau. Hemmer:Pfizer: Honoraria. Zhang:Bristol Myers Squibb: Research Funding; Astellas Pharma Inc: Research Funding; Gamida Cell: Research Funding. Zhang:Pfizer: Current Employment. Shah:Pfizer: Current Employment, Current holder of stock options in a privately-held company. Welch:Pfizer: Current Employment, Current equity holder in publicly-traded company. Tikka:Pfizer: Current Employment, Current equity holder in publicly-traded company. Vandendries:Pfizer: Current Employment, Current equity holder in publicly-traded company. Stelljes:MSD: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Medac: Honoraria; Jazz: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Amgen: Consultancy; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Marks:Pfizer: Consultancy, Honoraria, Speakers Bureau; Kite: Consultancy, Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal